-------------------------------------------------------------------------------------------------
----------------------------------------------------------------------------------------------------
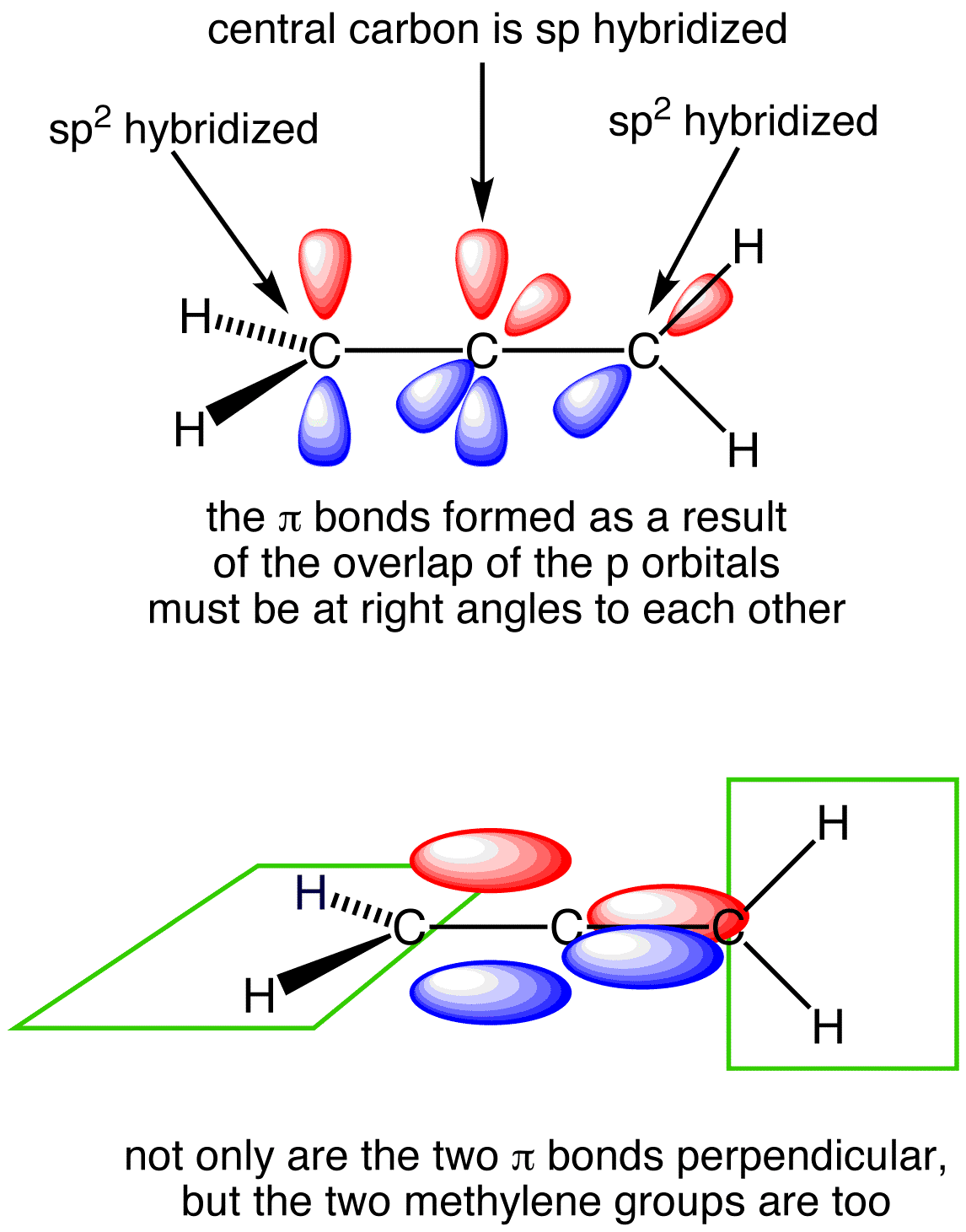
Look at the following illustration to help understand delocalized pi bonding in molecules that have resonance structures. Picutre (a) shows a 2-D molecular skeleton. Picutre (b) shows the skeleton with unhybridized p orbitals perpendicular to plane of molecule. Picutre (c) shows "merged" p orbitals above/below the plane of the molecule, with delocalized electrons shared over the whole molecular instead of just between two atoms. And picture (d) showing the electron cloud (made up of the regular covalently bonded electrons in their hybrid orbitals, as well as the delocalized electrons) surrounding whole molecule, with electron density shown by color.
 |

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.