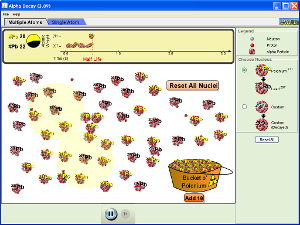
Watch these videos to help you gain a better understanding of Pressure and how gases mixed together affect it, as well as how the mass of gas molecules effects their speed of movement.
Monday, December 16, 2013
Gases -- Ideal Gas Law and Real Gases
Watch these videos to help you gain a better understanding of the Ideal Gas Law, how it works in problem solving, and how Real Gases deviate from the Ideal.
Thursday, December 12, 2013
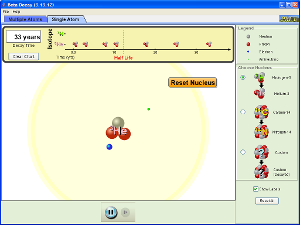
Types of Nuclear Reactions and Nuclear Equations
Watch the following videos to help you understand the different types of nuclear reactions that can occur and the nuclear equations we can write down to describe them.
Flame Test Lab
Watch the following video to see what we did in class when we performed the Flame Test Lab. Follow along and fill in the data table on your lab paper, including the answers for the two "unknown" chemicals. When this is done, please answer the lab questions that go along with the experiment, based on what you see in lab/the video and what we have learned in class about excited electrons and light.
Monday, December 9, 2013
Bonding #6 -- Polar vs. Non-Polar Molecules
Watch the following video for a good overview of Molecular Polarity, as well as Inter-Molecular Forces that result between molecules due to their polarities. (total time = 11 minutes)
Bonding #7 -- Resonance Stuctures and Delocalized Bonding of Electrons in Covalent Molecules
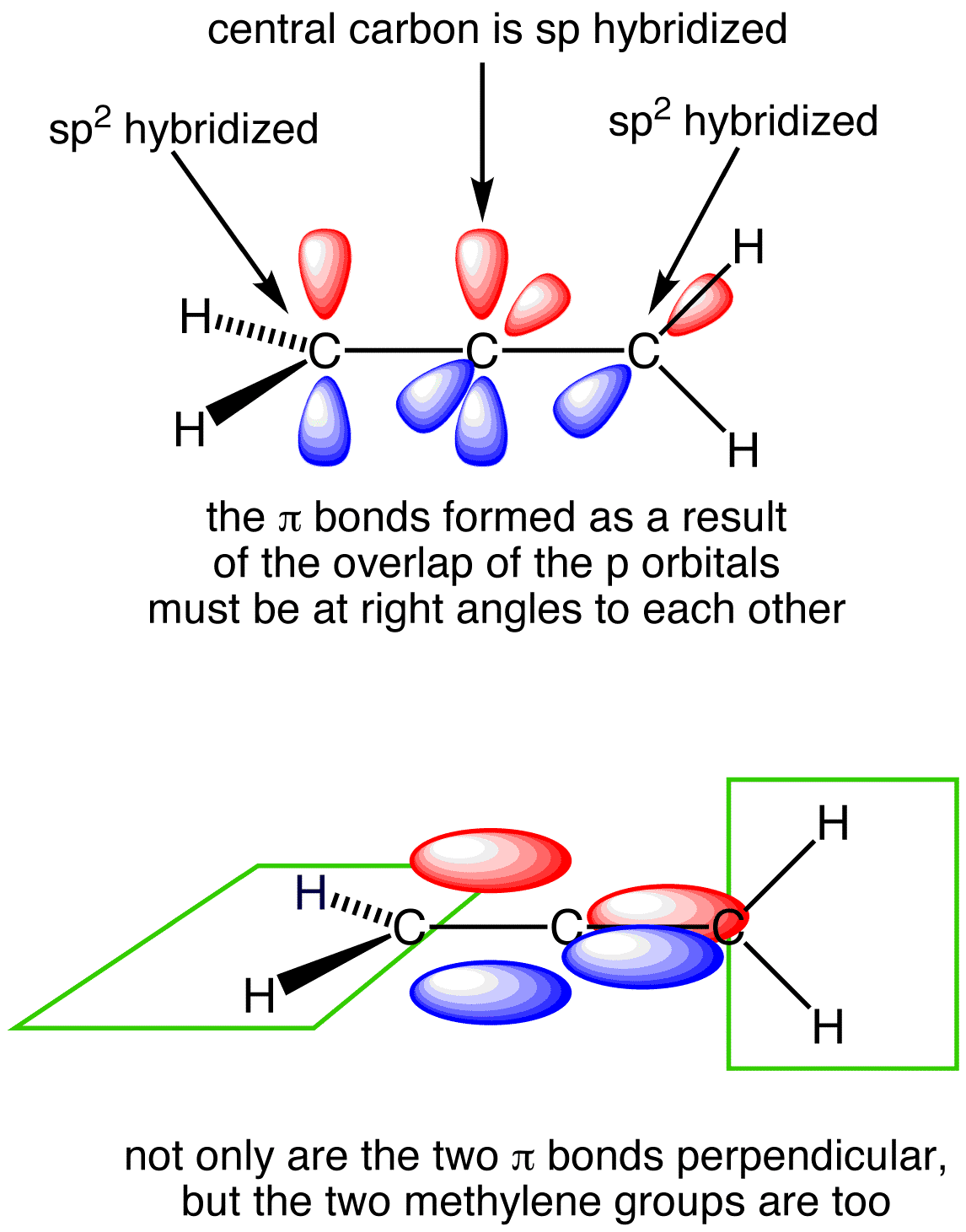
Read the following text and illustrations to help understand what resonance Lewis Structures actually mean in terms of real molecules. They involve delocalized bonding, or electrons that are not just shared between two atoms like in a normal ("localized") covalent bond, BUT instead are shared over three or more atoms/across the whole molecule.
-------------------------------------------------------------------------------------------------
----------------------------------------------------------------------------------------------------
Look at the following illustration to help understand delocalized pi bonding in molecules that have resonance structures. Picutre (a) shows a 2-D molecular skeleton. Picutre (b) shows the skeleton with unhybridized p orbitals perpendicular to plane of molecule. Picutre (c) shows "merged" p orbitals above/below the plane of the molecule, with delocalized electrons shared over the whole molecular instead of just between two atoms. And picture (d) showing the electron cloud (made up of the regular covalently bonded electrons in their hybrid orbitals, as well as the delocalized electrons) surrounding whole molecule, with electron density shown by color.

-------------------------------------------------------------------------------------------------

----------------------------------------------------------------------------------------------------
Look at the following illustration to help understand delocalized pi bonding in molecules that have resonance structures. Picutre (a) shows a 2-D molecular skeleton. Picutre (b) shows the skeleton with unhybridized p orbitals perpendicular to plane of molecule. Picutre (c) shows "merged" p orbitals above/below the plane of the molecule, with delocalized electrons shared over the whole molecular instead of just between two atoms. And picture (d) showing the electron cloud (made up of the regular covalently bonded electrons in their hybrid orbitals, as well as the delocalized electrons) surrounding whole molecule, with electron density shown by color.
 |

Bonding #5 -- Molecular Shapes and Geometry
Watch the following three videos for a good overview of Molecular Geometry and Shapes, including hybrid orbitals. (total time = 27 minutes) Also click on the two links for extra information about molecular shapes and hybrid orbitals.
Click on this link to learn about hybrid orbitals.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/hybrv18.swf
Click on this link for information about actual pictures of real molecules with shapes that match up with VSEPR and hybrid orbital theories!
http://phys.org/news/2013-05-first-ever-high-resolution-images-molecule-reforms.html
Click on this link to learn about hybrid orbitals.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/hybrv18.swf
Click on this link for information about actual pictures of real molecules with shapes that match up with VSEPR and hybrid orbital theories!
http://phys.org/news/2013-05-first-ever-high-resolution-images-molecule-reforms.html
Bonding #0 -- Bonding Overview
Watch the following video for a good overview of Chemical Bonding, especially as it relates to Coulumb's Law. (total time = 10 minutes)
Bonding #4 -- Bond Polarity
Watch the following video for a good overview of Electronegativity and Bond Polarity -- the difference between Ionic Bonds, Polar Covalent Bonds, and Non-Polar Covalent Bonds. (total time = 9 minutes)
Bonding #3 -- Covalent Bonding, Lewis Structures, VSEPR
Watch the following two videos for a good overview of Covalent Bonding, Lewis Diagrams, and the VSEPR model to explain molecular shapes.
Bonding #2 -- Metallic Bonding
Watch the following two videos for a good overview of Metallic Bonding and the properties of substances held together by metallic bonds, called Metals or Metallic Solids. (total time = 10 minutes)
Bonding #1 -- Ionic Bonding
Watch the following two videos for a good overview of Ionic Bonding and the properties of substances held together by ionic bonds, called Ionic Solids. (total time = 10 minutes)
Tuesday, November 19, 2013
Periodic Table Properties and Trends
Elements have different properties based on the valence electrons in their atoms. These valence electrons and properties control how the atoms of that element will react with other elements.
Because the number and position of valence electrons an atom has matches up with its location on the Periodic Table, elements with similar valence electrons will have similar properties and are found in the same groups (up and down columns) on the Periodic Table. In addition, as you go across each row from left to right and down each column on the Table, the elements show patterns in their properties and behaviors based on where they are located in the Table.
Several important properties of atoms we need to know about are:


http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/atomic4.swf

Because the number and position of valence electrons an atom has matches up with its location on the Periodic Table, elements with similar valence electrons will have similar properties and are found in the same groups (up and down columns) on the Periodic Table. In addition, as you go across each row from left to right and down each column on the Table, the elements show patterns in their properties and behaviors based on where they are located in the Table.
Several important properties of atoms we need to know about are:
- Atomic Size (Radius)
- Ionic Size (Radius)
- Ionization Energy
- Electronegativity
- Atomic Size (Radius) shown as a line graph.

- Atomic Size (Radius) shown as a bar graph in the Periodic Table.

- Click on this link to go to a Flash animation explaining about atomic properties, how they are related to electrons, and the patterns they show in the Periodic Table.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/atomic4.swf
- All of the properties and their patterns on the Periodic Table.

Thursday, November 7, 2013
Electrons and PES
PES = Photo-Electron Spectroscopy is a special technique used to determine the location and number of electrons in an atom based on their energy. It is another way of thinking about quantum numbers and it is experimental proof that electron configurations are accurate representations of the location and distribution of electrons within an atom.
Watch the following videos to get an idea of how the technique works and what the graphical results look like. Then notice how we can use these graphs to get information about the electron configuration and identities of atoms.

Watch the following videos to get an idea of how the technique works and what the graphical results look like. Then notice how we can use these graphs to get information about the electron configuration and identities of atoms.
- This second video has a worksheet that goes along with it that you will be asked to complete while watching the video.
- Lastly, read the diagram and text below AND the webpage shown in this link to help you understand what is going on with electrons in 3d and 4s, which orbtial they ACTUALLY fill in first, why they do it in that order, and why we learn electron configurations in the other order.
Electron-electron repulsion

It takes 1312 kJ of energy to remove the electron from a mole of hydrogen atoms. What might we expect this value to be for helium? Helium contains two electrons, but its nucleus contains two protons; each electron "sees" both protons, so we might expect that the electrons of helium would be bound twice as strongly as the electron of hydrogen. The ionization energy of helium should therefore be twice 1312 kJ/mol, or 2612 kJ/mol.
However, if one looks at the spectrum of helium, the continuum is seen to begin at a wavelength corresponding to an ionization energy of 2372 kJ/mol, or about 90% of the predicted value.
Why are the electrons in helium bound less tightly than the +2 nuclear charge would lead us to expect?
The answer is that there is another effect to consider: the repulsion between the two electrons; the resulting electron-electron repulsion subtracts from the force holding the electron to the nucleus, reducing the local binding of each.
Electron-electron repulsion is a major factor in both the spectra and chemical behavior of the elements heavier than hydrogen.
Unpaired Electrons: Paramagnetism and Diamagnetism
Watch the two videos that follow to help you understand the difference between paramagnetism and diamagnetism, as well as how each phenomenon is caused by unpaired and paired electrons in atomic orbitals. Also, check out the liquid oxygen in the second video! (Total time = 10 minutes)
Tuesday, November 5, 2013
Electrons #5: Electrons in Atoms and Periodicity
Electron Configurations use quantum numbers (probability descriptions of where an electron is located outside of its nucleus) to differentiate among and list out all of the electrons in an atom.
http://www.sciencegeek.net/Chemistry/taters/Unit2ElectronNotations.htm
http://www.sciencegeek.net/Chemistry/taters/Unit3ValenceElectrons.htm
- Watch this short video and then go to each of the website links that follow. Pay extra attention to the discussion of Coulomb's Law and shielding.
- Answer the questions on each of the links.
http://www.sciencegeek.net/Chemistry/taters/Unit2ElectronNotations.htm
http://www.sciencegeek.net/Chemistry/taters/Unit3ValenceElectrons.htm
Sunday, November 3, 2013
Electrons #4: Electronic Orbitals, Part II
Watch the following videos to learn about and help you understand the electronic structure of atoms. (Total time 30 minutes)
- 1st video goes into more detail about what the quantum numbers we use to describe where electrons are in each atom actually mean.
- 2nd video continues to discuss quantum numbers and uses more pictures of orbitals to help you understand how we have to talk about the electrons in an atom.
Saturday, November 2, 2013
Electrons #3: Electronic Orbitals, Part I
Watch the following video to learn about and help you understand the electronic structure of atoms.
http://www.chemguide.co.uk/atoms/properties/atomorbs.html

- 1st video has a good overview of the whole process.
- 2nd video explains the techniques and vocabulary chemists use to talk about electrons and their strange behavior as they move around the nucleus.
- Click on the following link to read about electron orbitals and electron configurations.
http://www.chemguide.co.uk/atoms/properties/atomorbs.html
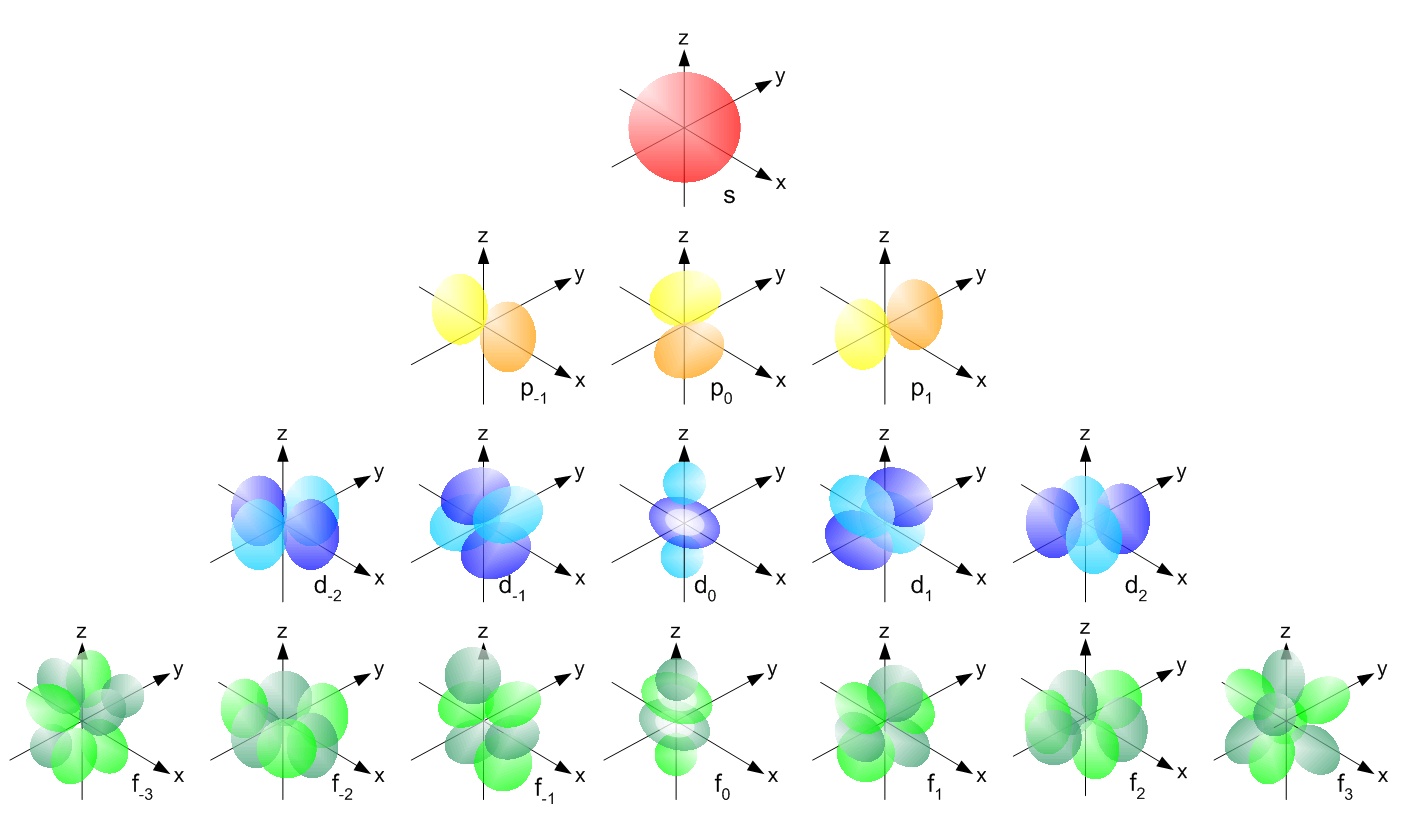
- Look at the following two pictures. The first is a simplified color-coded representation of the s, p, d, and f sublevels and the 1, 3, 5, and 7 orientations (orbtials) that each one has respectively. The second picture shows some of the same orbitals as they really are, probability density plots of the electron's wave function.


Friday, November 1, 2013
Electrons #2: Electrons and Light
Click on this link and follow along with the Flash animation to help you understand how the quantum nature of electrons was discovered by looking at the colors of light produced when elements are heated. The animation also connects the colors of light produced to the location and behavior of electrons in an atom, relative to their nucleus.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/linesp16.swf
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/linesp16.swf
Thursday, October 31, 2013
Electrons #1: Quantum Nature of Electrons
After watching the NOVA special in class, watch the following videos (the first video links to a playlist of 8 short videos, totaling 40 minutes) to help you understand quantum physics and how it applies to electrons.
Wednesday, October 30, 2013
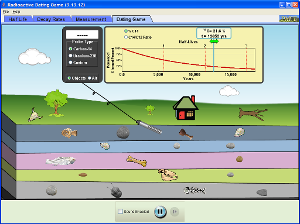
Half-Life and Radioactive Decay Activities
Look at the following graph and run the three applets on this page to explore and learn more about radioactive decay and half-life.
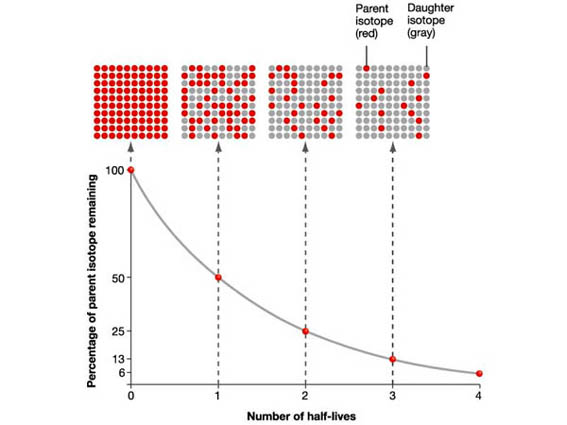 |
| Half-Life: Radioactive Decay and Exponential Curve |
The first applet shows alpha decay.
The third applet shows half-life curves and how to use them to find the age of things buried in the ground.
Radioactive Decay and Half-Life Overview
Watch the following video to learn more about radioactive decay and how long it takes atoms to decay, known as half-life.
Watch the following video to help you understand how to do half-life calculations.
Watch the following video to help you understand how to do half-life calculations.
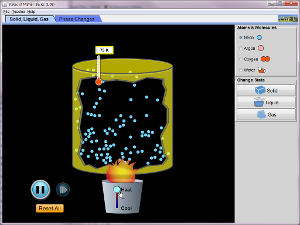
Thermochemistry -- Phase Changes (temperature only)
Run the following applet to explore how adding heat energy to a sample changes temperature, state of matter, and molecular motion.
Thursday, October 17, 2013
Calorimetery--Calculating the Calorimeter Constant
Click on this link to watch a a short video that explains how to calculate the calorimeter constant (how "leaky" your calorimeter is when it comes to heat) using a sample problem.
http://www.chemteam.info/Thermochem/Video-calorimeter-constant-02.mov
http://www.chemteam.info/Thermochem/Video-calorimeter-constant-02.mov
Nuclear Chemistry -- Radiation and Radioactive Decay
Watch the following videos and click on the link below it to help learn about radioactive decay and the types of particles and radiation that are involved.
Alpha, Beta and Gamma Radiation Compared
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/radioa7.swf
Alpha, Beta and Gamma Radiation Compared
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/radioa7.swf
Tuesday, October 8, 2013
Thermochemistry Part II, the Math and the Experiments
Watch the following videos to help you understand the math sections of thermochemistry, both theoretical and experimental.
total time = 23 minutes
Enthalpy -- heat without work
Calorimetry -- heat experiments
total time = 23 minutes
Enthalpy -- heat without work
Calorimetry -- heat experiments
Thermochemistry Part I, the Concepts
Watch the following videos to help you understand the aspects of thermochemistry.
total viewing time = 14 minutes
Basic Concepts and Vocabulary
Energy, Heat, and Work
total viewing time = 14 minutes
Basic Concepts and Vocabulary
Energy, Heat, and Work
Periodic Table
Watch the following video to learn about the history of how the periodic table was developed.
Click on the following links to go to two websites with interactive Periodic Tables. Work with these and they will help you to understand what we need to know about the modern Periodic Table -- how it is arranged, what the different sections of it are called, and important properties of specific elements and groups of elements.
http://www.learner.org/interactives/periodic/index.html
http://www.ptable.com/#
Click on the following links to go to two websites with interactive Periodic Tables. Work with these and they will help you to understand what we need to know about the modern Periodic Table -- how it is arranged, what the different sections of it are called, and important properties of specific elements and groups of elements.
http://www.learner.org/interactives/periodic/index.html
http://www.ptable.com/#
Ernest Rutherford's Gold Foil Experiment
Click on the link below to work with an interactive animation and learn more about Ernest Rutherford's Gold Foil experiment that helped him to determine that atoms have a nucleus in the middle.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/ruther14.swf
Watch this video as well to help you understand the Gold Foil experiment.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/ruther14.swf
Watch this video as well to help you understand the Gold Foil experiment.
Monday, September 30, 2013
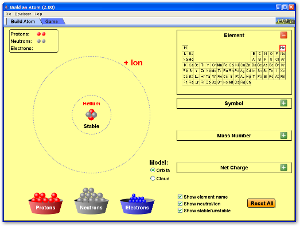
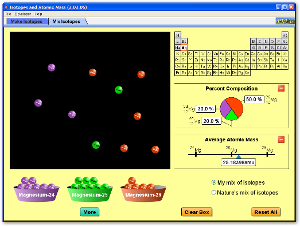
Atomic Structure -- Ions, Isotopes, and Weighted Average Atomic Mass
Use the following two Java applets to explore the idea of how the number of protons, neutrons, and electrons in an atom controls whether it is an ion, a stable isotope, or an unstable radioactive isotope.
Atoms and Atomic Structure
Watch the following videos for an introduction to and review of the basics of atomic structure, with some information about the history of how the atom was discovered and the Periodic Table as well.
Tuesday, September 24, 2013
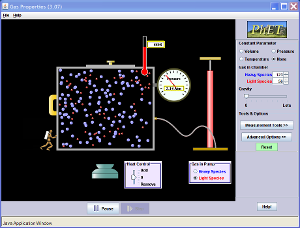
Gases Overall Review
Use the following video and Java applet to help reinforce your understanding of how the molecular-level view of gases controls their macro-scale behaviors.
Friday, September 20, 2013
Gases: Charles' Law (temperature and volume)
Follow the links below to watch two short videos to help you understand Charles' Law and the basic ideas of how gases behave on the molecular level.
total time = 13.5 minutes
http://education-portal.com/academy/lesson/temperature-units-converting-between-kelvins-and-celsius.html
http://education-portal.com/academy/lesson/charles-law-gas-pressure-and-temperature-relationship.html
total time = 13.5 minutes
http://education-portal.com/academy/lesson/temperature-units-converting-between-kelvins-and-celsius.html
http://education-portal.com/academy/lesson/charles-law-gas-pressure-and-temperature-relationship.html
Gases: Boyle's Law (pressure and volume)
Follow the links below to watch two short videos to help you understand Boyle's Law and the basic ideas of how gases behave on the molecular level.
total time = 13 minutes
http://education-portal.com/academy/lesson/pressure-and-temperature-conversions.html
http://education-portal.com/academy/lesson/boyles-charles-gay-lussacs-laws-pressure-volume-and-temperature-relationships.html
total time = 13 minutes
http://education-portal.com/academy/lesson/pressure-and-temperature-conversions.html
http://education-portal.com/academy/lesson/boyles-charles-gay-lussacs-laws-pressure-volume-and-temperature-relationships.html
Oxidation-Reduction Reactions
Watch the following videos to get an introduction to the topic of Oxidation-Reduction or Redox reactions. (23 minutes total)
Thursday, September 19, 2013
First Common Task in Chemistry -- Identifying Unknowns Lab Report
This week, the students have been working on an experiment in class. They have now completed the experiment, their calculations, their graphs, and their conclusions.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report must be types and can either be printed out and handed in OR emailed to me at my school email address mcnultyr@bsd-ri.net
The lab report is due next Friday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report must be types and can either be printed out and handed in OR emailed to me at my school email address mcnultyr@bsd-ri.net
The lab report is due next Friday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
First Common Task for Chemistry -- Identifying Unknowns Lab Report
This week, the students have been working on an experiment in class. They have now completed the experiment, their calculations, their graphs, and their conclusions.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report must be types and can either be printed out and handed in OR emailed to me at my school email address mcnultyr@bsd-ri.net
The lab report is due next Thursday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report is due next Thursday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
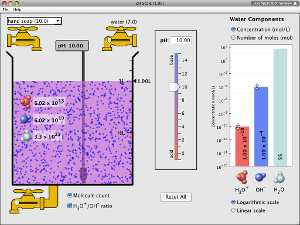
Acid-Base Titration Overview
Watch the following videos for an overview of acid-base titration lab technique, calculations, and graph.
Behavior of Gases on the Molecular Level
Follow the link below to watch a short video to help you understand the basic ideas of how gases behave on the molecular level.
total time = 7 minutes
http://education-portal.com/academy/lesson/the-kinetic-molecular-theory-properties-of-gases.html
Click on the link below to watch and work with two flash animations to help you understand how gases (as well as solids and liquids) behave at the molecular level.
http://preparatorychemistry.com/KMT_flash.htm
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/gasesv6.swf
total time = 7 minutes
http://education-portal.com/academy/lesson/the-kinetic-molecular-theory-properties-of-gases.html
Click on the link below to watch and work with two flash animations to help you understand how gases (as well as solids and liquids) behave at the molecular level.
http://preparatorychemistry.com/KMT_flash.htm
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/gasesv6.swf
Monday, September 16, 2013
Acid-Base Fundamentals
Watch the following videos, go to the website shown in the link, and work with the Java applet below to help you understand the special solutions known as acids and bases. (17.5 minutes total)
Go to this website and work with the flash animation to see the difference between strong and weak acids.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/acid13.swf
Go to this website and work with the flash animation to see the difference between strong and weak acids.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/acid13.swf
Additional Solution Videos
Watch the following videos to gain more understanding of solutions and solubility rules. (25 minutes total)
Tuesday, September 10, 2013
Chemistry Lab Equipment
Watch the following video to become familiar with the basic lab equipment and measurements devices we will use this year during chemistry experiments. Make sure you know the purpose of each piece of equipment and how to use the measurement devices correctly to get good quantitative data.
total time = 4:30 minutes
total time = 4:30 minutes
Graphing Data from an Experiment
When we take quantitative measurements during an experiment, this is called collecting data. Many times, we will graph this data to try to make sense of the numbers and figure out what the data is telling us about the results of our experiment.
Make sure you can create a graph and read an existing graph to get information from it about an experiment.
Total time = 10 minutes
Total time = 10 minutes
Quantitative Measurements in Experiments
Total time = 25 minutes
When we take quantitative measurements in chemistry, we will be using lab equipment, such as the scale or the graduated cylinder. All of the equipment we use will be set in the Metric System -- make sure you remember how to use this measurement system.
Watch the following video and check out the website listed below.
http://www.mathsisfun.com/measure/metric-system.html
When we take measurements, we must make sure that they are both accurate and precise. What do these terms mean?
How does this general discussion of accuracy and precision relate to using a scale or a graduated cylinder to measure chemicals in an experiment? Watch the following video that discusses these ideas in the context of the Uncertainty of your experimental measurements.
When we take quantitative measurements in chemistry, we will be using lab equipment, such as the scale or the graduated cylinder. All of the equipment we use will be set in the Metric System -- make sure you remember how to use this measurement system.
Watch the following video and check out the website listed below.
http://www.mathsisfun.com/measure/metric-system.html
When we take measurements, we must make sure that they are both accurate and precise. What do these terms mean?
How does this general discussion of accuracy and precision relate to using a scale or a graduated cylinder to measure chemicals in an experiment? Watch the following video that discusses these ideas in the context of the Uncertainty of your experimental measurements.
Types of Properties -- Extensive vs. Intensive and Qualitative vs. Quantitative
We must observe and measure the properties of the chemicals we work with in lab in order to find out what they are. We have already talked about Physical and Chemical, but there are other differences to consider as well. Watch the following videos that explain the differences between Extensive and Intensive, and then between Qualitative and Quantitative.
Total Time = 6 minutes
Monday, September 9, 2013
Chapter 4 -- Quantitative Aspects of Solutions
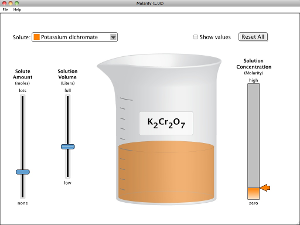
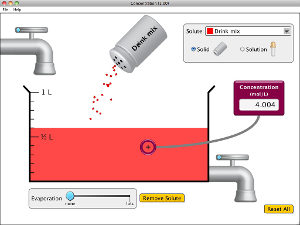
Run and explore both of these Java applets to help you understand the unit of Molarity and the concepts of concentration in solutions, such as saturated and unsaturated. Watch the video that follows for an excellent demonstration of how supersaturated solutions are unstable.
Chapter 4 - Solutions and the Solvation Process
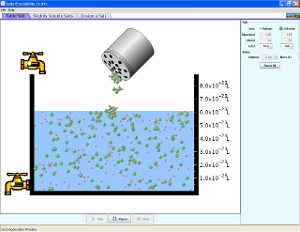
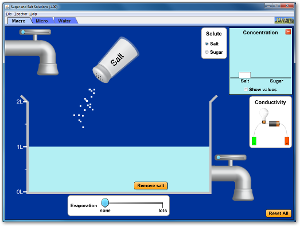
Run and explore all four of these Java applets to help you understand how solutes dissolve in solutions.
Click on the following link to go to the first applet.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/molvie1.swf
Click on the following link to go to the second applet.
http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/thermochem/solutionSalt.html
Click on the following link to go to the first applet.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/molvie1.swf
Click on the following link to go to the second applet.
http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/thermochem/solutionSalt.html
Chapter 4, part I -- Solutions and Net Ionic Equations
Watch these two videos for an introduction to Chapter 4 on the general topics of Types of Chemical Reactions and Solution Stoichiometry.
Total length = 20 minutes
Total length = 20 minutes
Friday, September 6, 2013
AP Chemistry Test on Chapters 1, 2, and 3
Make sure that you study for your test on Monday.
Use the review papers that I gave you in class and the videos on this blog.
Email me with questions if you have any at mcnultyr@bsd-ri.net
Use the review papers that I gave you in class and the videos on this blog.
Email me with questions if you have any at mcnultyr@bsd-ri.net
Wednesday, September 4, 2013
Virtual Labs for Introductory Topics
Follow these links to find the two virtual labs we will be performing.
Lab 1 = Chromatography Lab (Separating Mixtures)
http://bioweb.wku.edu/courses/Biol114/Psynth/Psynth1.asp
Lab 2 = Percent Composition and Empirical Formula of Hydrate
http://elearning.classof1.com/demo/3d_lab/EmpiricalFormula.html
Lab 1 = Chromatography Lab (Separating Mixtures)
http://bioweb.wku.edu/courses/Biol114/Psynth/Psynth1.asp
- complete first page only
- be prepared to answer questions in class discussion about this laboratory technique
Lab 2 = Percent Composition and Empirical Formula of Hydrate
http://elearning.classof1.com/demo/3d_lab/EmpiricalFormula.html
- complete the whole experiment for one hydrate, assigned by your teacher
- share your results with the other students in the class until everyone has data for all 5 hydrates
- answer all the questions and complete all sections in the lab packet
Tuesday, September 3, 2013
Separating Mixtures
Total time for both videos = 6:30 minutes
Substances are made of just one kind of particle so they CANNOT be physically separated into different components.
Mixtures are made of MORE than one kind of particle so they CAN be physically separated into their components. The first video below has a simple example of separating a mixtures using the different properties of the two components that were mixed together.
The second video describes the formal lab techniques that are used to separate the types of mixtures you would work with during a chemistry experiment.
Substances are made of just one kind of particle so they CANNOT be physically separated into different components.
Mixtures are made of MORE than one kind of particle so they CAN be physically separated into their components. The first video below has a simple example of separating a mixtures using the different properties of the two components that were mixed together.
The second video describes the formal lab techniques that are used to separate the types of mixtures you would work with during a chemistry experiment.
Stoichiometry -- Guided Problem Solving
These videos follow along with the NMSI packet we received in class. They work through problems from the packet step-by-step. Feel free to fast forward and skip over parts you do not need top review.
The first video covers problems on Average Atomic Mass, Molar Conversions, Molar Mass, Mass Percent (% composition), and Determining Empirical and Molecular Formulas.
The second video covers problems on Balancing Equations, Reaction Stoichiometry, Limiting Reactants, and Percent Yield.
AP Chemistry: Reaction Stoichiometry from Rene McCormick on Vimeo.
The first video covers problems on Average Atomic Mass, Molar Conversions, Molar Mass, Mass Percent (% composition), and Determining Empirical and Molecular Formulas.
The second video covers problems on Balancing Equations, Reaction Stoichiometry, Limiting Reactants, and Percent Yield.
AP Chemistry: Reaction Stoichiometry from Rene McCormick on Vimeo.
What is the Difference Between Physical and Chemical Changes?
Watch the following videos to refresh your memory about the differences between physical and chemical changes in matter and how we tell them apart from one another based on observations. Total viewing time = 15 minutes.
What type of matter is it and How do I know?
Watch the following videos to refresh your memory about the different types of matter that make up the universe around us and how we tell them apart from one another based on their properties. Total viewing time = 6 minutes.
Monday, September 2, 2013
Introduction to Stoichiometry
Watch the following video to learn about the basics of Stoichiometry. (about 13 minutes)
Friday, August 30, 2013
Lab Safety Assignments and Due Dates
By Friday, August 30th, students will receive a Science Safety Contract that we will review in class, along with the safety features of my lab classroom.
They will then be responsible for reading, filling in, and signing this contract.
Please make sure that the parents/guardians of each student also reads and signs this safety contract.
Students will not be able to participate in any experiments until this signed contract is returned to me.
Return the signed contract by Tuesday, September 3rd.
There will be a Safety Quiz based on this safety contract and the lesson in class that students must pass to be able to participate in experiments in class. This quiz will be on Tuesday, September 3th.
There will also be a Chemical Safety Poster assignment that goes along with this, and it must be completed outside of class and handed in by Friday, September 6th.
They will then be responsible for reading, filling in, and signing this contract.
Please make sure that the parents/guardians of each student also reads and signs this safety contract.
Students will not be able to participate in any experiments until this signed contract is returned to me.
Return the signed contract by Tuesday, September 3rd.
There will be a Safety Quiz based on this safety contract and the lesson in class that students must pass to be able to participate in experiments in class. This quiz will be on Tuesday, September 3th.
There will also be a Chemical Safety Poster assignment that goes along with this, and it must be completed outside of class and handed in by Friday, September 6th.
Thursday, August 29, 2013
Homework Thursday 08/29/13
Homework for today -- practice Safety Quiz
Look at each illustration, figure out all the things that the people are doing that are wrong or UNSAFE, and write them on the lines underneath each picture.
Students will be called to write their answers on the board tomorrow at the start of class.
Wednesday, August 28, 2013
First Few Days of Chemistry Class
As an introductory activity in class on the first day, students will be investigating the differences between physical and chemical changes -- a review of topics covered in middle school and earlier high school science classes.
Over the first few days of school, students will be getting a course outline with important information about what they need for class each day, how grades are assigned in my classes, and other useful things.
They will also be receiving a sheet on the rules of my classroom -- a bit different because it is a laboratory classroom with the extra concerns of lab equipment and chemicals to consider.
Tuesday, August 27, 2013
Welcome to Mr. McNulty's Chemistry Classes 2013-2014!
Hello and Welcome to all of my students and their parents/guardians!
This blog will contain helpful information, videos, applets, websites, etc. that will connect with the topics and assignments that we cover in chemistry class.
Information and dates about homework, lab reports, tests and quizzes, and other class assignments will be posted as well.
If you ever have questions or concerns that are not addressed on this blog, please feel free to contact me at my school email, mcnultyr@bsd-ri.net
This blog will contain helpful information, videos, applets, websites, etc. that will connect with the topics and assignments that we cover in chemistry class.
Information and dates about homework, lab reports, tests and quizzes, and other class assignments will be posted as well.
If you ever have questions or concerns that are not addressed on this blog, please feel free to contact me at my school email, mcnultyr@bsd-ri.net
Thursday, June 13, 2013
Questions? Find the Answers here!
Questions about your Chemistry class? Not sure what the homework is tonight? When is that lab report due? Check out my blog, Holy Flaming Peanuts! for all the answers.
Subscribe to:
Posts (Atom)