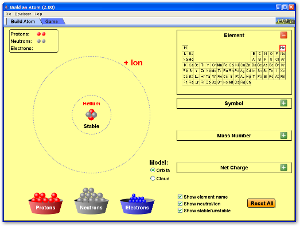
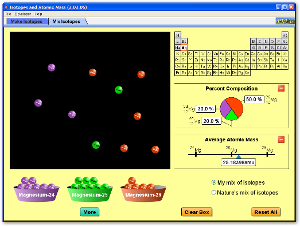
Use the following two Java applets to explore the idea of how the number of protons, neutrons, and electrons in an atom controls whether it is an ion, a stable isotope, or an unstable radioactive isotope.
Monday, September 30, 2013
Atoms and Atomic Structure
Watch the following videos for an introduction to and review of the basics of atomic structure, with some information about the history of how the atom was discovered and the Periodic Table as well.
Tuesday, September 24, 2013
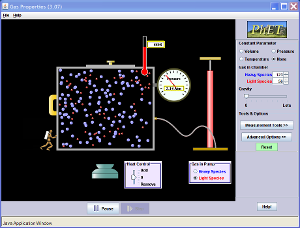
Gases Overall Review
Use the following video and Java applet to help reinforce your understanding of how the molecular-level view of gases controls their macro-scale behaviors.
Friday, September 20, 2013
Gases: Charles' Law (temperature and volume)
Follow the links below to watch two short videos to help you understand Charles' Law and the basic ideas of how gases behave on the molecular level.
total time = 13.5 minutes
http://education-portal.com/academy/lesson/temperature-units-converting-between-kelvins-and-celsius.html
http://education-portal.com/academy/lesson/charles-law-gas-pressure-and-temperature-relationship.html
total time = 13.5 minutes
http://education-portal.com/academy/lesson/temperature-units-converting-between-kelvins-and-celsius.html
http://education-portal.com/academy/lesson/charles-law-gas-pressure-and-temperature-relationship.html
Gases: Boyle's Law (pressure and volume)
Follow the links below to watch two short videos to help you understand Boyle's Law and the basic ideas of how gases behave on the molecular level.
total time = 13 minutes
http://education-portal.com/academy/lesson/pressure-and-temperature-conversions.html
http://education-portal.com/academy/lesson/boyles-charles-gay-lussacs-laws-pressure-volume-and-temperature-relationships.html
total time = 13 minutes
http://education-portal.com/academy/lesson/pressure-and-temperature-conversions.html
http://education-portal.com/academy/lesson/boyles-charles-gay-lussacs-laws-pressure-volume-and-temperature-relationships.html
Oxidation-Reduction Reactions
Watch the following videos to get an introduction to the topic of Oxidation-Reduction or Redox reactions. (23 minutes total)
Thursday, September 19, 2013
First Common Task in Chemistry -- Identifying Unknowns Lab Report
This week, the students have been working on an experiment in class. They have now completed the experiment, their calculations, their graphs, and their conclusions.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report must be types and can either be printed out and handed in OR emailed to me at my school email address mcnultyr@bsd-ri.net
The lab report is due next Friday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report must be types and can either be printed out and handed in OR emailed to me at my school email address mcnultyr@bsd-ri.net
The lab report is due next Friday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
First Common Task for Chemistry -- Identifying Unknowns Lab Report
This week, the students have been working on an experiment in class. They have now completed the experiment, their calculations, their graphs, and their conclusions.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report must be types and can either be printed out and handed in OR emailed to me at my school email address mcnultyr@bsd-ri.net
The lab report is due next Thursday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
They should have all of the information they need to complete a Lab Report on this experiment. They have received written instructions on how to write up this experiment as well as an in-class review of exactly what I am looking for in this lab report.
This is a very important assignment. It counts as a test grade for 1st quarter AND as 25% of their Mid-Year Exam grade! Please make sure that the student completes and hands in this lab report on time.
The lab report is due next Thursday, September 26th. If the students have any questions, need any help, or would like me to review a rough draft of the lab report before they hand it in, they may come to see me after school for extra help any day.
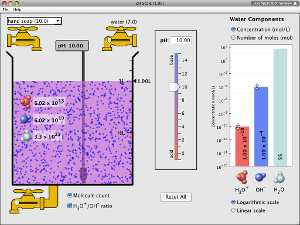
Acid-Base Titration Overview
Watch the following videos for an overview of acid-base titration lab technique, calculations, and graph.
Behavior of Gases on the Molecular Level
Follow the link below to watch a short video to help you understand the basic ideas of how gases behave on the molecular level.
total time = 7 minutes
http://education-portal.com/academy/lesson/the-kinetic-molecular-theory-properties-of-gases.html
Click on the link below to watch and work with two flash animations to help you understand how gases (as well as solids and liquids) behave at the molecular level.
http://preparatorychemistry.com/KMT_flash.htm
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/gasesv6.swf
total time = 7 minutes
http://education-portal.com/academy/lesson/the-kinetic-molecular-theory-properties-of-gases.html
Click on the link below to watch and work with two flash animations to help you understand how gases (as well as solids and liquids) behave at the molecular level.
http://preparatorychemistry.com/KMT_flash.htm
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/gasesv6.swf
Monday, September 16, 2013
Acid-Base Fundamentals
Watch the following videos, go to the website shown in the link, and work with the Java applet below to help you understand the special solutions known as acids and bases. (17.5 minutes total)
Go to this website and work with the flash animation to see the difference between strong and weak acids.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/acid13.swf
Go to this website and work with the flash animation to see the difference between strong and weak acids.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/acid13.swf
Additional Solution Videos
Watch the following videos to gain more understanding of solutions and solubility rules. (25 minutes total)
Tuesday, September 10, 2013
Chemistry Lab Equipment
Watch the following video to become familiar with the basic lab equipment and measurements devices we will use this year during chemistry experiments. Make sure you know the purpose of each piece of equipment and how to use the measurement devices correctly to get good quantitative data.
total time = 4:30 minutes
total time = 4:30 minutes
Graphing Data from an Experiment
When we take quantitative measurements during an experiment, this is called collecting data. Many times, we will graph this data to try to make sense of the numbers and figure out what the data is telling us about the results of our experiment.
Make sure you can create a graph and read an existing graph to get information from it about an experiment.
Total time = 10 minutes
Total time = 10 minutes
Quantitative Measurements in Experiments
Total time = 25 minutes
When we take quantitative measurements in chemistry, we will be using lab equipment, such as the scale or the graduated cylinder. All of the equipment we use will be set in the Metric System -- make sure you remember how to use this measurement system.
Watch the following video and check out the website listed below.
http://www.mathsisfun.com/measure/metric-system.html
When we take measurements, we must make sure that they are both accurate and precise. What do these terms mean?
How does this general discussion of accuracy and precision relate to using a scale or a graduated cylinder to measure chemicals in an experiment? Watch the following video that discusses these ideas in the context of the Uncertainty of your experimental measurements.
When we take quantitative measurements in chemistry, we will be using lab equipment, such as the scale or the graduated cylinder. All of the equipment we use will be set in the Metric System -- make sure you remember how to use this measurement system.
Watch the following video and check out the website listed below.
http://www.mathsisfun.com/measure/metric-system.html
When we take measurements, we must make sure that they are both accurate and precise. What do these terms mean?
How does this general discussion of accuracy and precision relate to using a scale or a graduated cylinder to measure chemicals in an experiment? Watch the following video that discusses these ideas in the context of the Uncertainty of your experimental measurements.
Types of Properties -- Extensive vs. Intensive and Qualitative vs. Quantitative
We must observe and measure the properties of the chemicals we work with in lab in order to find out what they are. We have already talked about Physical and Chemical, but there are other differences to consider as well. Watch the following videos that explain the differences between Extensive and Intensive, and then between Qualitative and Quantitative.
Total Time = 6 minutes
Monday, September 9, 2013
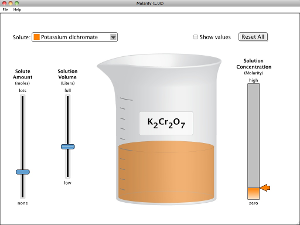
Chapter 4 -- Quantitative Aspects of Solutions
Run and explore both of these Java applets to help you understand the unit of Molarity and the concepts of concentration in solutions, such as saturated and unsaturated. Watch the video that follows for an excellent demonstration of how supersaturated solutions are unstable.
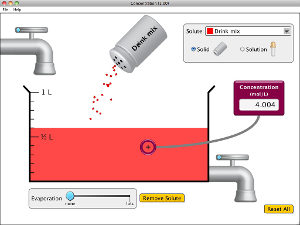
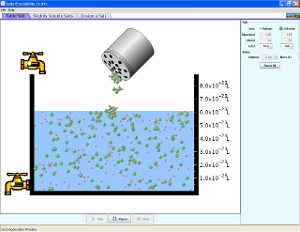
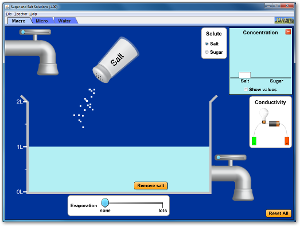
Chapter 4 - Solutions and the Solvation Process
Run and explore all four of these Java applets to help you understand how solutes dissolve in solutions.
Click on the following link to go to the first applet.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/molvie1.swf
Click on the following link to go to the second applet.
http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/thermochem/solutionSalt.html
Click on the following link to go to the first applet.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/molvie1.swf
Click on the following link to go to the second applet.
http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/thermochem/solutionSalt.html
Chapter 4, part I -- Solutions and Net Ionic Equations
Watch these two videos for an introduction to Chapter 4 on the general topics of Types of Chemical Reactions and Solution Stoichiometry.
Total length = 20 minutes
Total length = 20 minutes
Friday, September 6, 2013
AP Chemistry Test on Chapters 1, 2, and 3
Make sure that you study for your test on Monday.
Use the review papers that I gave you in class and the videos on this blog.
Email me with questions if you have any at mcnultyr@bsd-ri.net
Use the review papers that I gave you in class and the videos on this blog.
Email me with questions if you have any at mcnultyr@bsd-ri.net
Wednesday, September 4, 2013
Virtual Labs for Introductory Topics
Follow these links to find the two virtual labs we will be performing.
Lab 1 = Chromatography Lab (Separating Mixtures)
http://bioweb.wku.edu/courses/Biol114/Psynth/Psynth1.asp
Lab 2 = Percent Composition and Empirical Formula of Hydrate
http://elearning.classof1.com/demo/3d_lab/EmpiricalFormula.html
Lab 1 = Chromatography Lab (Separating Mixtures)
http://bioweb.wku.edu/courses/Biol114/Psynth/Psynth1.asp
- complete first page only
- be prepared to answer questions in class discussion about this laboratory technique
Lab 2 = Percent Composition and Empirical Formula of Hydrate
http://elearning.classof1.com/demo/3d_lab/EmpiricalFormula.html
- complete the whole experiment for one hydrate, assigned by your teacher
- share your results with the other students in the class until everyone has data for all 5 hydrates
- answer all the questions and complete all sections in the lab packet
Tuesday, September 3, 2013
Separating Mixtures
Total time for both videos = 6:30 minutes
Substances are made of just one kind of particle so they CANNOT be physically separated into different components.
Mixtures are made of MORE than one kind of particle so they CAN be physically separated into their components. The first video below has a simple example of separating a mixtures using the different properties of the two components that were mixed together.
The second video describes the formal lab techniques that are used to separate the types of mixtures you would work with during a chemistry experiment.
Substances are made of just one kind of particle so they CANNOT be physically separated into different components.
Mixtures are made of MORE than one kind of particle so they CAN be physically separated into their components. The first video below has a simple example of separating a mixtures using the different properties of the two components that were mixed together.
The second video describes the formal lab techniques that are used to separate the types of mixtures you would work with during a chemistry experiment.
Stoichiometry -- Guided Problem Solving
These videos follow along with the NMSI packet we received in class. They work through problems from the packet step-by-step. Feel free to fast forward and skip over parts you do not need top review.
The first video covers problems on Average Atomic Mass, Molar Conversions, Molar Mass, Mass Percent (% composition), and Determining Empirical and Molecular Formulas.
The second video covers problems on Balancing Equations, Reaction Stoichiometry, Limiting Reactants, and Percent Yield.
AP Chemistry: Reaction Stoichiometry from Rene McCormick on Vimeo.
The first video covers problems on Average Atomic Mass, Molar Conversions, Molar Mass, Mass Percent (% composition), and Determining Empirical and Molecular Formulas.
The second video covers problems on Balancing Equations, Reaction Stoichiometry, Limiting Reactants, and Percent Yield.
AP Chemistry: Reaction Stoichiometry from Rene McCormick on Vimeo.
What is the Difference Between Physical and Chemical Changes?
Watch the following videos to refresh your memory about the differences between physical and chemical changes in matter and how we tell them apart from one another based on observations. Total viewing time = 15 minutes.
What type of matter is it and How do I know?
Watch the following videos to refresh your memory about the different types of matter that make up the universe around us and how we tell them apart from one another based on their properties. Total viewing time = 6 minutes.
Monday, September 2, 2013
Introduction to Stoichiometry
Watch the following video to learn about the basics of Stoichiometry. (about 13 minutes)
Subscribe to:
Posts (Atom)