Watch these videos to help you gain a better understanding of Pressure and how gases mixed together affect it, as well as how the mass of gas molecules effects their speed of movement.
Monday, December 16, 2013
Gases -- Ideal Gas Law and Real Gases
Watch these videos to help you gain a better understanding of the Ideal Gas Law, how it works in problem solving, and how Real Gases deviate from the Ideal.
Thursday, December 12, 2013
Types of Nuclear Reactions and Nuclear Equations
Watch the following videos to help you understand the different types of nuclear reactions that can occur and the nuclear equations we can write down to describe them.
Flame Test Lab
Watch the following video to see what we did in class when we performed the Flame Test Lab. Follow along and fill in the data table on your lab paper, including the answers for the two "unknown" chemicals. When this is done, please answer the lab questions that go along with the experiment, based on what you see in lab/the video and what we have learned in class about excited electrons and light.
Monday, December 9, 2013
Bonding #6 -- Polar vs. Non-Polar Molecules
Watch the following video for a good overview of Molecular Polarity, as well as Inter-Molecular Forces that result between molecules due to their polarities. (total time = 11 minutes)
Bonding #7 -- Resonance Stuctures and Delocalized Bonding of Electrons in Covalent Molecules
Read the following text and illustrations to help understand what resonance Lewis Structures actually mean in terms of real molecules. They involve delocalized bonding, or electrons that are not just shared between two atoms like in a normal ("localized") covalent bond, BUT instead are shared over three or more atoms/across the whole molecule.
-------------------------------------------------------------------------------------------------
----------------------------------------------------------------------------------------------------
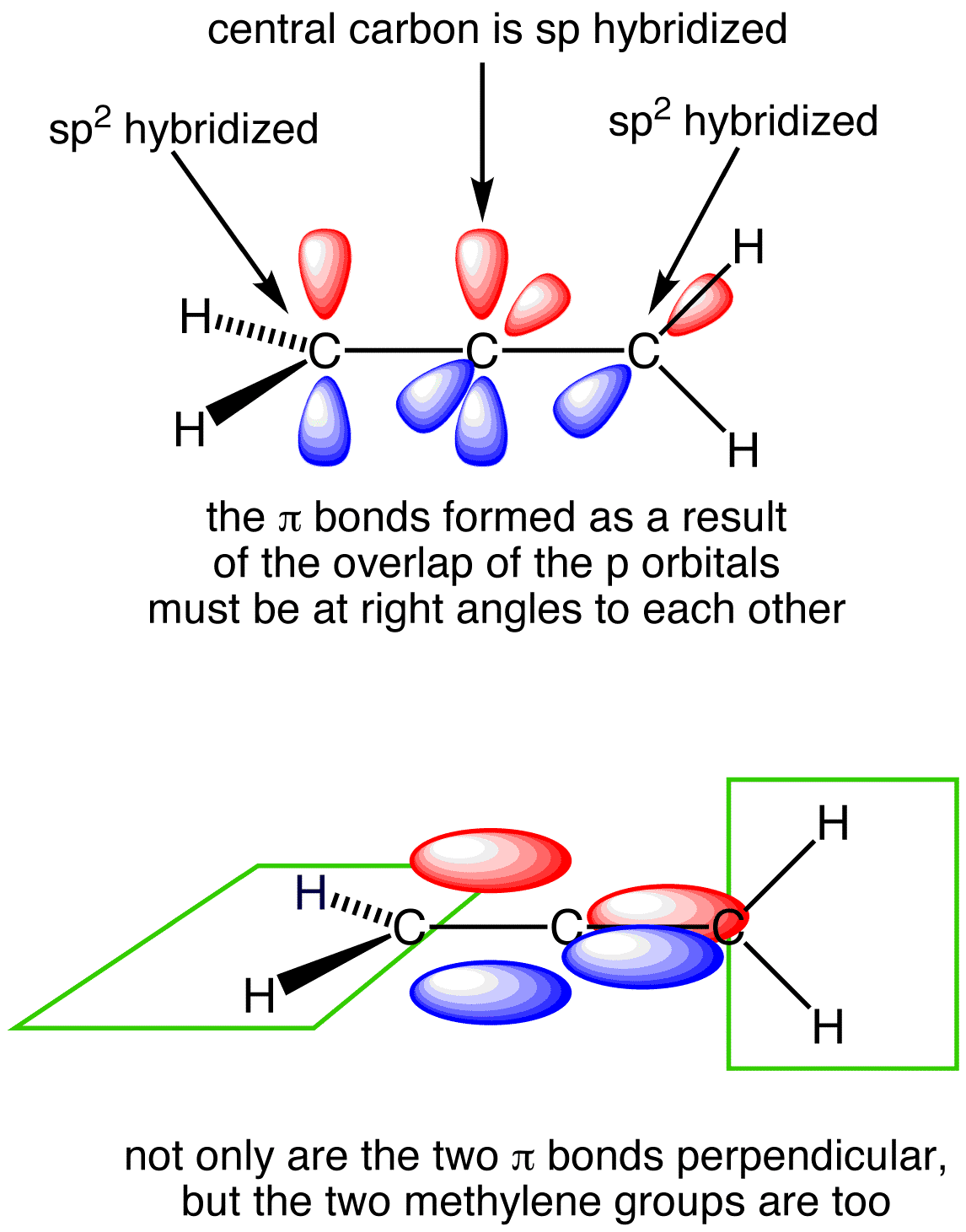
Look at the following illustration to help understand delocalized pi bonding in molecules that have resonance structures. Picutre (a) shows a 2-D molecular skeleton. Picutre (b) shows the skeleton with unhybridized p orbitals perpendicular to plane of molecule. Picutre (c) shows "merged" p orbitals above/below the plane of the molecule, with delocalized electrons shared over the whole molecular instead of just between two atoms. And picture (d) showing the electron cloud (made up of the regular covalently bonded electrons in their hybrid orbitals, as well as the delocalized electrons) surrounding whole molecule, with electron density shown by color.

-------------------------------------------------------------------------------------------------

----------------------------------------------------------------------------------------------------
Look at the following illustration to help understand delocalized pi bonding in molecules that have resonance structures. Picutre (a) shows a 2-D molecular skeleton. Picutre (b) shows the skeleton with unhybridized p orbitals perpendicular to plane of molecule. Picutre (c) shows "merged" p orbitals above/below the plane of the molecule, with delocalized electrons shared over the whole molecular instead of just between two atoms. And picture (d) showing the electron cloud (made up of the regular covalently bonded electrons in their hybrid orbitals, as well as the delocalized electrons) surrounding whole molecule, with electron density shown by color.
 |

Bonding #5 -- Molecular Shapes and Geometry
Watch the following three videos for a good overview of Molecular Geometry and Shapes, including hybrid orbitals. (total time = 27 minutes) Also click on the two links for extra information about molecular shapes and hybrid orbitals.
Click on this link to learn about hybrid orbitals.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/hybrv18.swf
Click on this link for information about actual pictures of real molecules with shapes that match up with VSEPR and hybrid orbital theories!
http://phys.org/news/2013-05-first-ever-high-resolution-images-molecule-reforms.html
Click on this link to learn about hybrid orbitals.
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/hybrv18.swf
Click on this link for information about actual pictures of real molecules with shapes that match up with VSEPR and hybrid orbital theories!
http://phys.org/news/2013-05-first-ever-high-resolution-images-molecule-reforms.html
Bonding #0 -- Bonding Overview
Watch the following video for a good overview of Chemical Bonding, especially as it relates to Coulumb's Law. (total time = 10 minutes)
Bonding #4 -- Bond Polarity
Watch the following video for a good overview of Electronegativity and Bond Polarity -- the difference between Ionic Bonds, Polar Covalent Bonds, and Non-Polar Covalent Bonds. (total time = 9 minutes)
Bonding #3 -- Covalent Bonding, Lewis Structures, VSEPR
Watch the following two videos for a good overview of Covalent Bonding, Lewis Diagrams, and the VSEPR model to explain molecular shapes.
Bonding #2 -- Metallic Bonding
Watch the following two videos for a good overview of Metallic Bonding and the properties of substances held together by metallic bonds, called Metals or Metallic Solids. (total time = 10 minutes)
Bonding #1 -- Ionic Bonding
Watch the following two videos for a good overview of Ionic Bonding and the properties of substances held together by ionic bonds, called Ionic Solids. (total time = 10 minutes)
Subscribe to:
Posts (Atom)